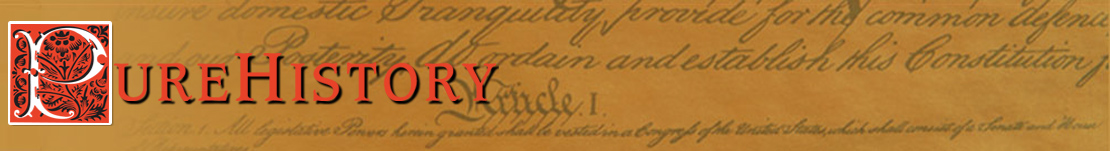The Nucleus
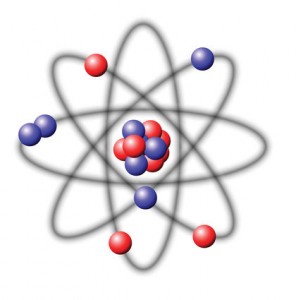
The Nucleus: Crash Course Chemistry #1
Hank does his best to convince us that chemistry is not torture, but is instead the amazing and beautiful science of stuff. Chemistry can tell us how three tiny particles – the proton, neutron and electron – come together in trillions of combinations to form … everything. In this inaugural episode of Crash Course Chemistry, we start out with one of the biggest ideas in chemistry ever – stuff is made from atoms. More specifically, we learn about the properties of the nucleus and why they are important to defining what an atom actually is.
Atomic Nucleus
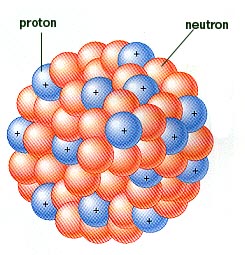
 The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911 as a result of Ernest Rutherford’s interpretation of the 1909 Geiger–Marsden gold foil experiment performed by Hans Geiger and Ernest Marsden under Rutherford’s direction. The proton–neutron model of nucleus was proposed by Dmitry Ivanenko in 1932. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud.
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911 as a result of Ernest Rutherford’s interpretation of the 1909 Geiger–Marsden gold foil experiment performed by Hans Geiger and Ernest Marsden under Rutherford’s direction. The proton–neutron model of nucleus was proposed by Dmitry Ivanenko in 1932. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud.
The diameter of the nucleus is in the range of 1.75 fm (1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).
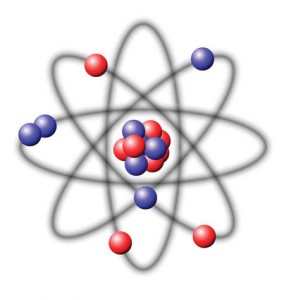
 The branch of physics concerned with studying and understanding the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.
The branch of physics concerned with studying and understanding the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.
History – Rutherford Model
The nucleus was discovered in 1911, as a result of Ernest Rutherford’s efforts to test Thomson’s “plum pudding model” of the atom. The electron had already been discovered earlier by J.J. Thomson himself, and knowing that atoms are neutral, Thomson postulated that there must be a positive charge as well. In his plum pudding model, Thomson stated that an atom consisted of negative electrons randomly scattered within a sphere of positive charge. Ernest Rutherford later devised an experiment that involved the deflection of alpha particles at a thin sheet of metal foil. He reasoned that if Thomson’s model were correct, the immense alpha particles would easily pass through the foil with very little deviation in their paths. To his surprise, many of the particles were deflected at very large angles. Because the mass of alpha particles is about 8000 times that of an electron, it became apparent that a very strong force was present that allowed the particles to be deflected. He realized that the plum pudding model could not be accurate and that the deflections of the alpha particles could only be caused by a center of concentrated charge that contained most of the atom’s mass. Thus, the idea of a nuclear atom—an atom with a dense center of positive charge—became justified.
Etymology
The term nucleus is from the Latin word nucleus, a diminutive of nux (“nut”), meaning the kernel (i.e., the “small nut”) inside a watery type of fruit (like a peach). In 1844, Michael Faraday used the term to refer to the “central point of an atom”. The modern atomic meaning was proposed by Ernest Rutherford in 1912. The adoption of the term “nucleus” to atomic theory, however, was not immediate. In 1916, for example, Gilbert N. Lewis stated, in his famous article The Atom and the Molecule, that “the atom is composed of the kernel and an outer atom or shell.”